
Their chemical formula can be written as XY 8( S, Se) 8 in which X is usually replaced by silver, manganese, cadmium, and lead, while copper takes the place of Y. The pentlandite group is a subdivision of rare minerals that share similar chemical and structural properties with pentlandite, hence the name. Pentlandite is synonymous with folgerite, horbachite, lillhammerite, and nicopyrite. It is chemically similar to mackinawite, godlevskite and horomanite. Pentlandite occurs alongside sulfide minerals such as bravoite, chalcopyrite, cubanite, millerite, pyrrhotite, valleriite, as well as other minerals like chromite, ilmenite, magnetite, and sperrylite. Photomicrograph showing flame-like pentlandite intergrowth in plane polarized light (PPL) (a) and cross polarized light (XPL) (b) (5x magnification, FOV = 4 mm) Mineral Associations Pentlandite usually develops as granular inclusions within other sulfide minerals (mainly pyrrhotite), often taking the shape of thin veins or "flames." Although pentlandite is an opaque mineral, it exhibits a strong light creamy reflectance. When looked at using reflected light ore microscopy, it possesses key diagnostic properties such as octahedral cleavage, and its alteration to bravoite, a pinkish to brownish violet sulfide mineral that occurs in euhedral to octahedral crystals. In contrast, pyrite, pyrrhotite and chalcopyrite will all display much darker streaks: brownish black, greyish black, greenish black respectively. For this reason, the best way to discern pentlandite is by its paler color, lack of magnetism, and light brownish bronze streak. In the field, pentlandite is often confused with other sulfide minerals, as they are all brassy yellowish in color and have a metallic luster. It is named after Irish scientist Joseph Barclay Pentland (1797–1873), who first noted the mineral at Sudbury, Ontario.Ĭopper Cliff mine, Sudbury, Ontario (1913) Identification Physical & Optical Properties It also occasionally occurs within mantle xenoliths and "black smoker" hydrothermal vents.

Pentlandite is found in abundance within ultramafic rocks, making it one of the most important sources of mined nickel. It has a yellowish bronze color and a metallic luster. It is brittle with a hardness of 3.5–4 and specific gravity of 4.6–5.0 and is non-magnetic. Pentlandite forms isometric crystals, but it is normally found in massive granular aggregates.
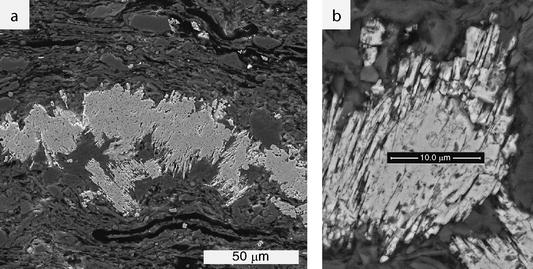
It also contains minor cobalt, usually at low levels as a fraction of weight. In some cases, this ratio is skewed by the presence of pyrrhotite inclusions. Pentlandite has a narrow variation range in nickel to iron ratios (Ni:Fe), but it is usually described as 1:1. Pentlandite is an iron– nickel sulfide with the chemical formula ( Fe, Ni) 9 S 8. Pentlandite in pyrrhotite, ore specimen from the Sudbury Basin (field of view 3.4 cm) Lo Narrator: Funded by Louisiana Board of Regents Contract No.3.1 x 2.6 cm mass of Pentlandite with some Pyrrhotite CLICK PAUSE END RECORDINGĢ Video ID: © 2008, Project VALUE (Video Assessment Library for Undergraduate Education), Department of Physical Sciences Nicholls State University Author: Glenn V. Note that we do not indicate the charges of the ions in the formula of a compound. Therefore, the formula should have subscripts to tell us that… in one formula unit… CLICK CLICK There are two cations… And three anions…. … and three sulfide ions… will have have a total charge of –6. Two iron three cations… will have a total charge of +6. In this case, the simplest combination consists of two cations and three anions. To write the formula for an ionic compound, we determine the simplest combination of ions with a total charge of zero. By now, you should know that members of this group form monatomic ions with a charge of –2. By referring to a periodic table, we find that sulfur belongs to group six A.

The roman numeral three tells us that the charge of the cation is +3. In an ionic compound, the cation is named first. We know it’s ionic because iron is a metallic element. CLICK Iron three sulfide is an ionic compound…. Fe3S2 PAUSE To name a compound given its formula, we need to first classify the compound, then apply the rule for that type of compound. Therefore: Cation is iron(III), or Fe3+ Anion is sulfide, or S2- Formula unit, the simplest combination of ions with a total charge of zero, consists of two cations and three anions x (+3) = x (-2) = -6 Cations: +3, +3, +3, +3, +3, … Anions: -2, -2, -2, -2, -2, … SCRIPT: Which of the following is the formula for iron(III) sulfide? A. Fe3S2 Iron(III) sulfide is an ionic compound. QUESTION: Which of the following is the formula for iron(III) sulfide? A. 1 Iron(III) sulfide is an ionic compound.


 0 kommentar(er)
0 kommentar(er)
